Publications
Group highlights
(For a full list of publications see below)
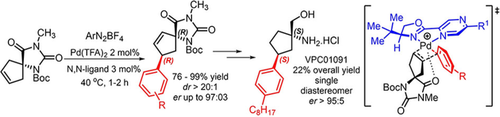
A highly efficient Heck-Matsuda desymmetrization of unsaturated spirohydantoins directed by non-covalent interactions, which allows the construction of two simultaneous stereogenic centers, including a trisubstituted quaternary one, is described. This Heck arylation permitted a novel enantioselective total synthesis of the S1PR1 agonist (also an S1PR3 antagonist) compound VPC01091, a potential drug for the treatment of multiple sclerosis. The broad scope of these enantioselective Heck-Matsuda protocol provided several arylated spiro systems in yields ranging from 76% to 99%, with enantiomeric ratios (er) up to 97:3, and diastereoselectivities (dr) of >20:1 in all cases studied. The method uses only 2% of Pd(TFA)2 and 3 mol% of the chiral N,N-ligand Pyrabox in short reaction times of 1–2 h. These enantioselective Heck arylations can also be carried out at the gram scale in high yields with no erosion of their diastereoselectivity or enantioselectivity. The key spiro Heck products (R,R)-21 and (R,R)-27 bearing an aryl iodide moiety or an aryl n-octyl moiety were employed as starting materials for the total enantioselective syntheses of the (S,S)-VPC01091, in overall yields of 20% and 22% respectively after 10 or 9 steps from the starting spirohydantoin, with an er>95:5. Computational analysis of the enantioselective Heck-Matsuda desymmetrization supports the rationale involving a key non-covalent interaction between the imide carbonyl of the spirohydantoin and the cationic palladium bounded to the chiral N,N-ligand.
V. C. de Oliveira, J. M. de Oliveira, V. H. Menezes da Silva, I. U. Khan, C. R. D. Correia
Adv. Synth. Catal. 2020, 362, 3395.
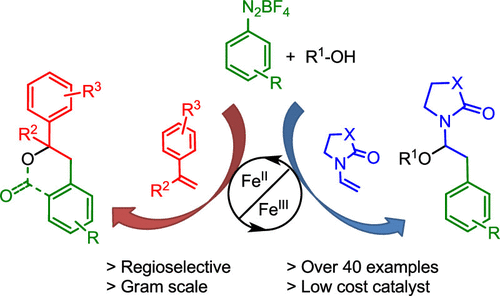
The arylative oxygenation of the electron-rich olefins styrene, α-methylstyrene, vinyl pyrrolidinone, and vinyl oxazolidinone was accomplished using arenediazonium salts and catalytic amounts of FeSO4 in an effective single electron transfer radical process. A broad range of aryldiazonium salts was tolerated using water, methanol, or their combination with acetonitrile to furnish the corresponding carbohydroxylated and carbomethoxylated products (42 examples), including functionalized dihydroisocoumarin and dihydrobenzofuran systems in good to excellent yields (up to 88%). The protocols developed for the Fe(II)-catalyzed carbohydroxylation were also compared to Ru(II) and Ir(III) photoredox carbooxygenations of these electron-rich olefins. The Fe(II)-catalyzed process proved to be highly competitive compared to the photoredox and the uncatalyzed processes. The proposed mechanism for the Fe(II)-catalyzed reactions involves the synergic combination with an effective Fe+2/Fe+3 redox system and a radical polar crossover mechanism featuring an unprecedented capture of the reactive N-acyliminium in the case of vinyl pyrrolidinone and vinyl oxazolidinone.
de Souza, E.L.S; Wiethan. C; Correia, C. R. D
ACS Omega 2019, 4, 20, 18918–18929.
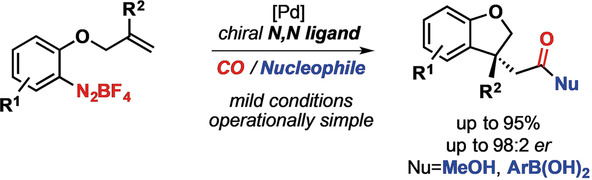
Unprecedented enantioselective intramolecular Heck carbonylation reactions of arenediazonium salts were enabled by a chiral N,N ligand. This reaction constitutes the first enantioselective Heck carbonylation that proceeds through migratory insertion followed by CO insertion. The enantioenriched functionalized dihydrobenzofurans were obtained in good to high yields and enantiomeric ratios of up to 98:2 under mild and operationally simple reaction conditions.
Carmona, R. C.; Köster, O. D.; Correia, C. R. D.
Angew. Chem. Int. Ed., 2018, 130, 12243-12246.
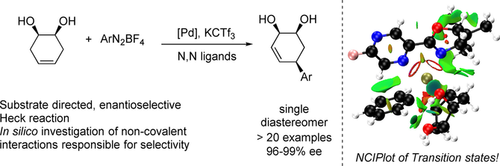
Enantioselective substrate directed Heck reactions are desirable for the stereoselective synthesis of complex molecules. However, due to the coordination requirements of both chiral ligands and directing groups, such methodologies are underdeveloped. We report herein the desymmetrization of meso (1R,2S)-cyclohex-4-ene-1,2-diol in an enantioselective and substrate directed fashion. The method provides all cis substituted highly functionalized chiral allylic alcohols in a complementary fashion to other Heck protocols.The products were obtained in high enantioselectivities (higher than 95% ee) and moderate to high yields (38–87%). The noncovalent interactions responsible for the directing effect were elucidated through computational examination of relevant minima and transition structures.
Angnes, R. A.; Thompson, L. M.; Mashuta, M. S.; Correia, C. R. D.; Hammond, G. B.,
Adv. Synth. Cat., 2018, 360, 3760-3767.
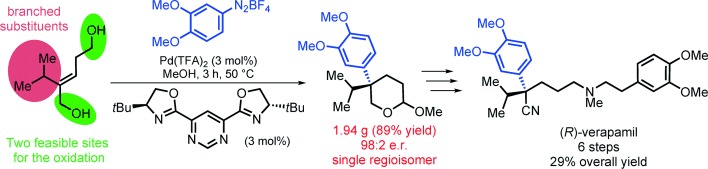
We describe herein a highly regio- and enantioselective Pd-catalyzed Heck arylation of unactivated trisubstituted acyclic olefins to provide all-carbon quaternary stereogenic centers. Chiral N,N ligands of the pyrimidine- and pyrazino-oxazoline class were developed for that purpose, providing the desired products in good to high yields with enantiomeric ratios up to >99:1. Both linear and branched substituents on the olefins were well-tolerated. The potential of this new method is demonstrated by the straightforward synthesis of several O-methyl lactols and lactones containing quaternary stereocenters, together with a concise enantioselective total synthesis of the calcium channel blocker verapamil.
Oliveira, C. C.; Pfaltz, A.; Correia, C. R. D.
Angew. Chem. Int. Ed. 2015, 54, 14036-14039.
Full List of publications
In Tandem Auto-Sustainable Enantioselective Heck-Matsuda Reactions Directly from Anilines
C. L. Herrera, J. V. Santiago, J. C. Pastre, C. R. D. Correia.
Adv. Synth. Catal. 2022, 364, 1863.
The Chemistry and Biological Applications of 3H-Pyrrolo[2,3-c]quinolines and Marinoquinolines
R. Dias do Espírito Santo, R. M. Capitão, P. Santos Barbosa, E. F. Simão dos Santos, C. Roque Duarte Correia.
Asian J. Org. Chem. 2021, 10, 1938.
Heck arylation of acyclic olefins employing arenediazonium salts and chiral N,N ligands: new mechanistic insights from quantum-chemical calculations
da Silva, V.H.M.; Oliveira, C.C.; Correia, C.R.D; Ataualpa, A.C.B.
Theor Chem Acc 139, 77 (2020).
Enantioselective Heck-Matsuda Reactions of Spirocyclopentenyl Hydantoins Directed by Non-Covalent Interactions: Total Synthesis of the (S,S)-VPC01091
V. C. de Oliveira, J. M. de Oliveira, V. H. Menezes da Silva, I. U. Khan, C. R. D. Correia
Adv. Synth. Catal. 2020, 362, 3395.
Enantioselective Heck Arylation of Acyclic Alkenol Aryl Ethers: Synthetic Applications and DFT Investigation of the Stereoselectivity
E. C. Polo, M. F. Wang, R. A. Angnes, A. A. C. Braga, C. R. D. Correia
Adv. Synth. Catal. 2020, 362, 884.
Iron-Catalyzed Meerwein Carbooxygenation of Electron-Rich Olefins: Studies with Styrenes, Vinyl Pyrrolidinone, and Vinyl Oxazolidinone
de Souza, E.L.S; Wiethan. C; Correia, C. R. D
ACS Omega 2019, 4, 20, 18918–18929.
DFT perspective on the selectivity and mechanism of ligand-free Heck reaction involving allylic esters and arenediazonium salts
Vitor H. Menezes da Silva, Nelson H. Morgon, Carlos R.D. Correia, Ataualpa A.C. Braga
Journal of Organometallic Chemistry, 896, 2019, 5-15
Enantioselective Synthesis of Phthalides and Isochromanones via Heck–Matsuda Arylation of Dihydrofurans
Kattela, S.; de Lucca, E. C., Jr. ; Correia, C. R. D
Chem. Eur. J., 2018, 24, 11691-11696.
N,N‐Chiral Ligands Enabling Palladium‐catalyzed Enantioselective Intramolecular Heck‐Matsuda Carbonylation Reactions by Sequential Migratory and CO Insertions
Carmona, R. C.; Köster, O. D.; Correia, C. R. D.
Angew. Chem. Int. Ed., 2018, 130, 12243-12246.
Non-Covalent Substrate Directed Enantioselective Heck Desymmetrization of cis-Cyclohex-4-ene-1,2-diol: Synthesis of All cis Chiral 5-Aryl-cyclohex-3-ene-1,2-diols and Mechanistic Investigation
Angnes, R. A.; Thompson, L. M.; Mashuta, M. S.; Correia, C. R. D.; Hammond, G. B.,
Adv. Synth. Cat., 2018, 360, 3760-3767.
Enantioselective, Noncovalent, Substrate-Directable Heck–Matsuda and Oxidative Heck Arylations of Unactivated Five-Membered Carbocyclic Olefins
Oliveira, J. M.; Angnes, R. A.; Khan, I. K.; Polo, E. C.; Heerdt, G.; Servilha, B. M.; Silva, V. H. M.; Braga, A. A. C.; Correia, C. R. D
Chem. Eur. J., 2018, 24, 11738-11747.
Regioselective and Stereoselective Heck–Matsuda Arylations of Trisubstituted Allylic Alkenols and Their Silyl and Methyl Ether Derivatives To Access Two Contiguous Stereogenic Centers: Expanding the Redox-Relay Process and Application in the Total Synthesis of meso-Hexestrol
Frota, C.; Polo, E. C.; Esteves, H.; Correia, C. R. D.
J. Org. Chem. 2018, 83, 2198–2209.
EnantioselectiveOxy-Heck–Matsuda Arylations:ExpeditiousSynthesis of Dihydrobenzofuran Systems and Total Synthesis ofthe Neolignan (-)-Conocarpan
Silva, A. R.; Polo, E. C.; Martins, N. M.; Correia, C. R. D.
Adv. Synth. Cat., 2018, 360, 346-365.
Non-Covalent Carbonyl-Directed Heck–Matsuda Desymmetrizations: Synthesis of Cyclopentene-Fused Spirooxindoles, Spirolactones, and Spirolactams
Kattela, S.; Heerdt, G.; Correia, C. R. D.
Adv. Synth. Cat., 2017, 260-267.
Enantioselective total synthesis of the highly selective sphingosine-1-receptor VPC01091 by the Heck desymmetrization of a non-activated cyclopentene-fused spiro-pyrrolidinone
Khan, I. U.; Kattela, S.; Hassan, A.; Correia, C. R. D.
Org. Biomol. Chem., 2016,14, 9476-9480.
Non-Covalent Substrate-Directed Enantioselective heck Reactions. Synthesis of S- and P-Stereogenic Heterocycles
de Azambuja, F.; Carmona, R. C.; Chorro, T.; Heerdt, G.; Correia, C. R. D.
Chem. Eur. J. 2016, 22, 11205-11209.
Intermolecular Noncovalent Hydroxy-Directed Enantioselective Heck Desymmetrization of Cyclopentenol: Computationally Driven Synthesis of Highly Functionalized cis-4-Arylcyclopentenol Scaffolds
Oliveira, J. S.; Angnes, R. A.; Silva, V. H. M.; Servilha, B. M.; Adeel, M.; Braga, A. A. C.; Aponick, A.; Correia, C. R. D.
J. Org. Chem. 2016, 81, 2010–2018.
Quaternary Stereogenic Centers through Enantioselective Heck Arylation of Acyclic Olefins with Aryldiazonium Salts: Application in a Concise Synthesis of (R)-Verapamil
Oliveira, C. C.; Pfaltz, A.; Correia, C. R. D.
Angew. Chem. Int. Ed. 2015, 54, 14036-14039.
Stereoselective Synthesis of 3-Hydroxy-4-arylcyclopentanones and 4-Arylcyclopentenones through a Heck–Matsuda Desymmetrization of meso cis-4-Cyclopentene-1,3-diol
Carmona, R. C. ; Correia, C. R. D.
Adv. Synth. Cat. 2015, 357, 2639-2643.
Finelli, F. G. ; Godoi, M. N. ; Correia, C. R. D.
J. Braz. Chem. Soc. 2015, 26, 910-915.
Chemo-, Regio- and Stereoselective Heck Arylation of Allylated Malonates: Mechanistic Insights by ESI-MS and Synthetic Application toward 5-Arylmethyl-γ-lactones
Oliveira, C. C. ; Marques, M. V. ; Godoi, M. N. ; Regiani, T. ; Santos, V. G. ; dos Santos E. A. F. ; Eberlin, M. N. ; Sá, M. M. ; Correia, C. R. D.
Org. Lett. 2014, 16, 5180-5183.
Improved synthesis of bioactive stilbene derivatives applying design of experiments to the Heck–Matsuda reaction
Perez, C. C. ; Pena, J. M. ; Correia, C. R. D.
New J. Chem. 2014, 38, 3933-3938.
Stereoselective Synthesis of Aryl Cyclopentene Scaffolds by Heck–Matsuda Desymmetrization of 3-Cyclopentenol
Angnes, R. A. ; Oliveira, J. M. ; O., Caio C. ; Martins, N. C. ; Correia, C. R. D.
Chem. Eur. J. 2014, 20, 13117-13121.
Fluorescence from bisaryl-substituted maleimide derivatives
Lauer, M. H.; Drekener, R. L.; Correia, C. R. D.; Gehlen, M. H.
Photochem. Photobiol. Sci. 2014, 13, 859-866.
Construction of 3-arylpropylamines using Heck arylations. The total synthesis of cinacalcet hydrochloride, alverine, and tolpropamine
Prediger, P.; Silva, A. R.; Correia, C. R. D.
Tetrahedron. 2014, 70, 3333-3341.
Expeditious Synthesis of the Marine Natural Products Prepolycitrin A and Polycitrins A and B through Heck Arylations
Canto, K.; Ribeiro, R. S.; Biajoli, A. F. P.; Correia, C. R. D.
Eur. J. Org Chem. 2013, 35, 8004-8013.
“Dba-free” palladium intermediates of the Heck–Matsuda reaction
Machado, A. H. L.; Milagre, H. M. S.; Eberlin, L. S.; Sabino A. A.; Correia, C. R. D.; Eberlin, M. N.
Org. Biomol. Chem. 2013,11, 3277-3281.
Ionic Liquids and the Heck Coupling Reaction: An Update
Prediger, P.; Genisson, Y.; Correia, C. R. D.
Curr. Org. Chem. 2013, 17, 238-256.
Intermolecular Enantioselective Heck–Matsuda Arylations of Acyclic Olefins: Application to the Synthesis of β-Aryl-γ-lactones and β-Aryl Aldehydes
Oliveira, C. C.; Angnes, R. A. ;Correia, C. R. D.
J. Org. Chem. 2013, 78, 4373−4385.
Synthesis of pentabromopseudilin and other arylpyrrole derivatives via Heck arylations
Schwalm, C. S.; de Castro, I. B. D.; Ferrari, J. ; de Oliveira, F. L.; Aparicio, R. ;Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 1660-1663.
The first examples of the enantioselective Heck Matsuda reaction: arylation of unactivated cyclic olefins using chiral bisoxazolines
Oliveira, C. C.; Salles, A. G.; Santos, E. A. F.; Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 3325-3328.
Heck-Matsuda Arylation as a Strategy to Access Kavalactones Isolated from Polygala sabulosa
Soldi, C.; Moro, A. V.; Pizzolatti, M. G.; Correia, C. R. D.
Eur. J. Org Chem. 2012, 3607-3616.
Divergent total synthesis of the natural antimalarial marinoquinolines A, B, C, E and unnatural analogues
Schwalm, C. S.; Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 4836-4840.
Palladium catalysed regioselective arylation of indoles, benzofuran and benzothiophene with aryldiazonium salts
Baijoli, A. F. P.; da Penha, E. T.; Correia, C. R. D.
RSC Advances 2012, 2, 11930.
Coumarins from Free ortho-Hydroxy Cinnamates by Heck-Matsuda Arylations: A Scalable Total Synthesis of (R)-Tolterodine
Barancelli, D. A.; Salles, A. G. ; Taylor, J. G.; Correia, C. R. D.
Org. Lett. 2012, 14, 6036-6039.
Stereoselective Arylation of Substituted Cyclopentenes by Substrate-Directable Heck Matsuda Reactions: A Concise Total Synthesis of the Sphingosine 1-Phosphate Receptor (S1P) Agonist VPC01091
Oliveira, C. C. ; dos Santos, E. A. F. ; Nunes, J. H. B.; Correia, C. R. D.
J. Org. Chem. 2012, 77, 8182-8190.
Evolution and Synthetic Applications of the Heck-Matsuda Reaction: The Return of Arenediazonium Salts to Prominence
Taylor, J. G.; Moro, A. V.; Correia, C. R. D.
Eur. J. Org Chem. 2011, 1403-1428.This revision article was in the top-25 most accessed in European Journal of Organic Chemistry in 2011-2012
Facile synthesis of symmetrical 3,3-diarylacrylates by a Heck-Matsuda reaction: an expedient route to biologically active indanones
Taylor, J. G.; Ribeiro, R. S.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 3861-3864.
Stereoselective Synthesis of Unsymmetrical β,β-Diarylacrylates by a Heck-Matsuda Reaction: Versatile Building Blocks for Asymmetric Synthesis of β,β-Diphenylpropanoates, 3-Aryl-indole, 4-Aryl-3,4-dihydro-quinolin-2-one and Formal Synthesis of (-)-Indatraline
Taylor, J. G.; Correia, C. R. D.
J. Org. Chem. 2011, 76, 857-869.
Substrate-Directable Heck Reactions with Arenediazonium Salts. The Regio- and Stereoselective Arylation of Allylamine Derivatives and Applications in the Synthesis of Naftifine and Abamines
Prediger, P.; Barbosa, L. F. ; Génisson, Y.; Correia, C. R. D.
J. Org. Chem. 2011, 76, 7737-7749.
Expeditious synthesis of 3,4-dihydroisocoumarins and phthalides using the Heck Matsuda reaction
da Penha, E. T. ; Forni, J. A. ; Biajoli, A. F. P.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 3861-3864.
Heck-Matsuda arylation of 2-hetero-substituted acrylates
de Azambuja, F.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 42-45.
Stereoselective Synthesis of Aza Analogues of Isoaltholactone and Goniothalesdiol - New Applications of the Heck-Matsuda Reaction
Moro, A. V.; dos Santos, M. R.; Correia, C. R. D.Eur.
J. Org. Chem. 2011, 7259-7270.
The first intramolecular Heck Matsuda reaction and its application in the syntheses of benzofurans and indoles
Siqueira, F. A.; Taylor, J. G.; Correia, C. R. D.
Tetrahedron Lett. 2010, 51, 2102-2105.
Synthesis of Novel Room Temperature Chiral Ionic Liquids. Application as Reaction Media for the Heck Arylation of Aza-endocyclic Acrylates
Pastre, J. C.; Genisson, Y.; Saffon, N.; Dandurand, J.; Correia, C. R. D.
J. Braz. Chem. Soc. 2010, 21, 821-836.
Arylation of β,γ-unsaturated lactones by a Heck-Matsuda reaction: an unexpected route to aryldiazene butenolides and pyridazinones
Taylor, J. G.; Correia, C. R. D.
Química Nova 2010, 33, 2070-2074.
The scope of the Heck arylation of enol ethers with arenediazonium salts: a new approach to the synthesis of flavonoids
Machado, A. H. L.; De Sousa, M.; Patto, D.; Azevedo, L.; Bombonato, F. I.; Correia, C. R. D.
Tetrahedron Lett. 2009, 50, 1222-1225.
Remarkable Electronic Effect on the Diastereoselectivity of the Heck Reaction of Methyl Cinnamate with Arenediazonium Salts: Formal Total Synthesis of (±)-Indatraline and (±)-Sertraline
Pastre, J. C.; Correia, C. R. D.
Adv. Synth. Cat. 2009, 351,1217-1223.
Highly Regio- and Stereoselective Heck Reaction of Allylic Esters with Arenediazonium Salts: Application to the Synthesis of Kavalactones
Moro, A. V.; Cardoso, F. S. P.; Correia, C. R. D.
Org. Lett. 2009, 11, 3642-3645.
Heck arylation of styrenes with arenediazonium salts: short, efficient, and stereoselective synthesis of resveratrol, DMU-212, and analogues
Moro, A. V.; Cardoso, Pereira, F. S.; Correia, C. R. D.
Tetrahedron Lett. 2008, 49, 5668-5671
Synthesis of conformationally restricted acetylcholine analogues. Comparing lipase-mediated resolution with simulated moving bed chromatography of arylated β-hydroxy-pyrrolidines
Barreto, R. L.; Carpes, M. J. S.; Santana, C. C.; Correia, C. R. D.
Tetrahedron: Asymmetry 2007, 18, 435-442.
Regio- and Stereoselective Heck Arylations of N -Carbomethoxy- L -3-Dehydroproline Methyl Ester with Arenediazonium Salts. Total Synthesis of Neuroexcitatory Aryl Kainoids
da Silva, K. P.; Godoi, M. N.; Correia, C. R. D.
Org. Lett. 2007, 9, 2815-2818.
Stereoselective Heck-Matsuda Arylations of Chiral Dihydrofurans with Arenediazonium Tetrafluoroborates: An Efficient Enantioselective Total Synthesis of (-)-Isoaltholactone
Meira, P.; Moro, A. V.; Correia, C. R. D.
Synthesis 2007, 2279-2286.
Heck Arylation of Maleic Anhydrides Using Arenediazonium Tetrafluoroborates. Synthesis of Mono and Diarylated Maleic Anhydrides and of the Marine Alkaloids Prepolycitrin A and Polycitrin A
Burtoloso, A. C. B.; Garcia, A. L. L.; Miranda, K. C.; Correia, C. R. D.
Synlett 2006, 18, 3145-3149.
Efficient Heck Arylations of Cyclic and Acyclic Acrylate Derivatives Using Arenediazonium Tetrafluoroborates. A New Synthesis of the Antidepressant Drug (±)-Paroxetine
Pastre, J. C.; Correia, C. R. D.
Org. Lett. 2006, 8, 1657-1660.
Synthesis of 4-Aryl-2-pyrrolidones and β-Aryl-γ-amino-butyric Acid (GABA) Analogues by Heck Arylation of 3-Pyrrolines with Arenediazonium Tetrafluoroborates. Synthesis of (±)-Rolipram on a Multigram Scale and Chromatographic Resolution by Semipreparative Chiral Simulated Moving Bed Chromatography
Garcia, A. L. L.; Carpes, M. J. S.; de Oca, A. C. B. M.; dos Santos, M. A. G.; Santana, C. C.; Correia, C. R. D.
J. Org. Chem. 2005, 70, 1050-1053
Probing the Mechanism of the Heck Reaction with Arene Diazonium Salts by Electrospray Mass and Tandem Mass Spectrometry
Sabino, A. A.; Machado, A. H. L.; Eberlin, M. N.; Correia, C. R. D.
Angew. Chem. Int. Ed. 2004, 43, 2514-2518
Probing the Stereoselectivity of the Heck Arylation of Endocyclic Enecarbamates with Diazonium Salts. Concise Syntheses of (2S, 5R)-Phenylproline Methyl Ester and Schramm’s C-Azanucleoside
Severino, E. A.; Costenaro, E. R.; Garcia, A. L. L.; Correia, C. R. D.
Org. Lett. 2003, 5, 305-308
Synthesis of Aryl Pyrrolizidines from Endocyclic Enecarbamates. Novel Applications of the Heck Arylation of 3-Pyrrolines Using Diazonium Salts
Montes de Oca, A. C. B.; Correia, C. R. D.
ARKIVOC 2003, 10, 390-403
Heck arylations of N-acyl-3-pyrroline an N-acyl-1,2,5,6-tetrahydropyridine with aryldiazonium salts. Short syntheses of aryl y-and -lactams, baclofen, homobaclofen and analogues
Carpes, M. J. S.; Correia, C. R. D.
Tetrahedron Lett. 2002, 43, 741-744
Heck Arylation of N-Boc-3-Pyrrolines and N-Boc-2-Pyrrolines with Diazonium Salts. Efficient Syntheses of Five-Membered 4-Aryl Endocyclic Enecarbamates and N-Boc-2,4-Diaryl 3-Pyrrolines
Carpes, M. J. S.; Correia, C. R. D.
Synlett 2000, 1037-1039.
Heck Arylation of Endocyclic Enecarbamates with Diazonium Salts. Improvements and a Concise Enantioselective Synthesis of (-)-Codonopsinine
Severino, E. A.; Correia, C. R. D.
Org. Lett. 2000, 2, 3039-3042
Heck Reaction of Endocyclic Enecarbamates with Diazonium Salts. Formal Enantioselective Synthesis of Alkaloids (-)-Codonopsine and (-)-Codonopsinine, and the Synthesis of a New C-aryl Aza-Sugar
Oliveira, D. F.; Severino, E. A.; Correia, C. R. D.
Tetrahedron Lett. 1999, 40, 2083-2086
Enantioselective Synthesis of Phthalides and Isochromanones via Heck–Matsuda Arylation of Dihydrofurans
Kattela, S.; de Lucca, E. C., Jr. ; Correia, C. R. D
Chem. Eur. J., 2018, 24, 11691-11696.
N,N‐Chiral Ligands Enabling Palladium‐catalyzed Enantioselective Intramolecular Heck‐Matsuda Carbonylation Reactions by Sequential Migratory and CO Insertions
Carmona, R. C.; Köster, O. D.; Correia, C. R. D.
Angew. Chem. Int. Ed., 2018, 130, 12243-12246.
Non-Covalent Substrate Directed Enantioselective Heck Desymmetrization of cis-Cyclohex-4-ene-1,2-diol: Synthesis of All cis Chiral 5-Aryl-cyclohex-3-ene-1,2-diols and Mechanistic Investigation
Angnes, R. A.; Thompson, L. M.; Mashuta, M. S.; Correia, C. R. D.; Hammond, G. B.,
Adv. Synth. Cat., 2018, 360, 3760-3767.
Enantioselective, Noncovalent, Substrate-Directable Heck–Matsuda and Oxidative Heck Arylations of Unactivated Five-Membered Carbocyclic Olefins
Oliveira, J. M.; Angnes, R. A.; Khan, I. K.; Polo, E. C.; Heerdt, G.; Servilha, B. M.; Silva, V. H. M.; Braga, A. A. C.; Correia, C. R. D
Chem. Eur. J., 2018, 24, 11738-11747.
Regioselective and Stereoselective Heck–Matsuda Arylations of Trisubstituted Allylic Alkenols and Their Silyl and Methyl Ether Derivatives To Access Two Contiguous Stereogenic Centers: Expanding the Redox-Relay Process and Application in the Total Synthesis of meso-Hexestrol
Frota, C.; Polo, E. C.; Esteves, H.; Correia, C. R. D.
J. Org. Chem. 2018, 83, 2198–2209.
EnantioselectiveOxy-Heck–Matsuda Arylations:ExpeditiousSynthesis of Dihydrobenzofuran Systems and Total Synthesis ofthe Neolignan (-)-Conocarpan
Silva, A. R.; Polo, E. C.; Martins, N. M.; Correia, C. R. D.
Adv. Synth. Cat., 2018, 360, 346-365.
Non-Covalent Carbonyl-Directed Heck–Matsuda Desymmetrizations: Synthesis of Cyclopentene-Fused Spirooxindoles, Spirolactones, and Spirolactams
Kattela, S.; Heerdt, G.; Correia, C. R. D.
Adv. Synth. Cat., 2017, 260-267.
Enantioselective total synthesis of the highly selective sphingosine-1-receptor VPC01091 by the Heck desymmetrization of a non-activated cyclopentene-fused spiro-pyrrolidinone
Khan, I. U.; Kattela, S.; Hassan, A.; Correia, C. R. D.
Org. Biomol. Chem., 2016,14, 9476-9480.
Non-Covalent Substrate-Directed Enantioselective heck Reactions. Synthesis of S- and P-Stereogenic Heterocycles
de Azambuja, F.; Carmona, R. C.; Chorro, T.; Heerdt, G.; Correia, C. R. D.
Chem. Eur. J. 2016, 22, 11205-11209.
Intermolecular Noncovalent Hydroxy-Directed Enantioselective Heck Desymmetrization of Cyclopentenol: Computationally Driven Synthesis of Highly Functionalized cis-4-Arylcyclopentenol Scaffolds
Oliveira, J. S.; Angnes, R. A.; Silva, V. H. M.; Servilha, B. M.; Adeel, M.; Braga, A. A. C.; Aponick, A.; Correia, C. R. D.
J. Org. Chem. 2016, 81, 2010–2018.
Quaternary Stereogenic Centers through Enantioselective Heck Arylation of Acyclic Olefins with Aryldiazonium Salts: Application in a Concise Synthesis of (R)-Verapamil
Oliveira, C. C.; Pfaltz, A.; Correia, C. R. D.
Angew. Chem. Int. Ed. 2015, 54, 14036-14039.
Stereoselective Synthesis of 3-Hydroxy-4-arylcyclopentanones and 4-Arylcyclopentenones through a Heck–Matsuda Desymmetrization of meso cis-4-Cyclopentene-1,3-diol
Carmona, R. C. ; Correia, C. R. D.
Adv. Synth. Cat. 2015, 357, 2639-2643.
Finelli, F. G. ; Godoi, M. N. ; Correia, C. R. D.
J. Braz. Chem. Soc. 2015, 26, 910-915.
Chemo-, Regio- and Stereoselective Heck Arylation of Allylated Malonates: Mechanistic Insights by ESI-MS and Synthetic Application toward 5-Arylmethyl-γ-lactones
Oliveira, C. C. ; Marques, M. V. ; Godoi, M. N. ; Regiani, T. ; Santos, V. G. ; dos Santos E. A. F. ; Eberlin, M. N. ; Sá, M. M. ; Correia, C. R. D.
Org. Lett. 2014, 16, 5180-5183.
Improved synthesis of bioactive stilbene derivatives applying design of experiments to the Heck–Matsuda reaction
Perez, C. C. ; Pena, J. M. ; Correia, C. R. D.
New J. Chem. 2014, 38, 3933-3938.
Stereoselective Synthesis of Aryl Cyclopentene Scaffolds by Heck–Matsuda Desymmetrization of 3-Cyclopentenol
Angnes, R. A. ; Oliveira, J. M. ; O., Caio C. ; Martins, N. C. ; Correia, C. R. D.
Chem. Eur. J. 2014, 20, 13117-13121.
Fluorescence from bisaryl-substituted maleimide derivatives
Lauer, M. H.; Drekener, R. L.; Correia, C. R. D.; Gehlen, M. H.
Photochem. Photobiol. Sci. 2014, 13, 859-866.
Construction of 3-arylpropylamines using Heck arylations. The total synthesis of cinacalcet hydrochloride, alverine, and tolpropamine
Prediger, P.; Silva, A. R.; Correia, C. R. D.
Tetrahedron. 2014, 70, 3333-3341.
Expeditious Synthesis of the Marine Natural Products Prepolycitrin A and Polycitrins A and B through Heck Arylations
Canto, K.; Ribeiro, R. S.; Biajoli, A. F. P.; Correia, C. R. D.
Eur. J. Org Chem. 2013, 35, 8004-8013.
“Dba-free” palladium intermediates of the Heck–Matsuda reaction
Machado, A. H. L.; Milagre, H. M. S.; Eberlin, L. S.; Sabino A. A.; Correia, C. R. D.; Eberlin, M. N.
Org. Biomol. Chem. 2013,11, 3277-3281.
Ionic Liquids and the Heck Coupling Reaction: An Update
Prediger, P.; Genisson, Y.; Correia, C. R. D.
Curr. Org. Chem. 2013, 17, 238-256.
Intermolecular Enantioselective Heck–Matsuda Arylations of Acyclic Olefins: Application to the Synthesis of β-Aryl-γ-lactones and β-Aryl Aldehydes
Oliveira, C. C.; Angnes, R. A. ;Correia, C. R. D.
J. Org. Chem. 2013, 78, 4373−4385.
Synthesis of pentabromopseudilin and other arylpyrrole derivatives via Heck arylations
Schwalm, C. S.; de Castro, I. B. D.; Ferrari, J. ; de Oliveira, F. L.; Aparicio, R. ;Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 1660-1663.
The first examples of the enantioselective Heck Matsuda reaction: arylation of unactivated cyclic olefins using chiral bisoxazolines
Oliveira, C. C.; Salles, A. G.; Santos, E. A. F.; Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 3325-3328.
Heck-Matsuda Arylation as a Strategy to Access Kavalactones Isolated from Polygala sabulosa
Soldi, C.; Moro, A. V.; Pizzolatti, M. G.; Correia, C. R. D.
Eur. J. Org Chem. 2012, 3607-3616.
Divergent total synthesis of the natural antimalarial marinoquinolines A, B, C, E and unnatural analogues
Schwalm, C. S.; Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 4836-4840.
Palladium catalysed regioselective arylation of indoles, benzofuran and benzothiophene with aryldiazonium salts
Baijoli, A. F. P.; da Penha, E. T.; Correia, C. R. D.
RSC Advances 2012, 2, 11930.
Coumarins from Free ortho-Hydroxy Cinnamates by Heck-Matsuda Arylations: A Scalable Total Synthesis of (R)-Tolterodine
Barancelli, D. A.; Salles, A. G. ; Taylor, J. G.; Correia, C. R. D.
Org. Lett. 2012, 14, 6036-6039.
Stereoselective Arylation of Substituted Cyclopentenes by Substrate-Directable Heck Matsuda Reactions: A Concise Total Synthesis of the Sphingosine 1-Phosphate Receptor (S1P) Agonist VPC01091
Oliveira, C. C. ; dos Santos, E. A. F. ; Nunes, J. H. B.; Correia, C. R. D.
J. Org. Chem. 2012, 77, 8182-8190.
Evolution and Synthetic Applications of the Heck-Matsuda Reaction: The Return of Arenediazonium Salts to Prominence
Taylor, J. G.; Moro, A. V.; Correia, C. R. D.
Eur. J. Org Chem. 2011, 1403-1428.This revision article was in the top-25 most accessed in European Journal of Organic Chemistry in 2011-2012
Facile synthesis of symmetrical 3,3-diarylacrylates by a Heck-Matsuda reaction: an expedient route to biologically active indanones
Taylor, J. G.; Ribeiro, R. S.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 3861-3864.
Stereoselective Synthesis of Unsymmetrical β,β-Diarylacrylates by a Heck-Matsuda Reaction: Versatile Building Blocks for Asymmetric Synthesis of β,β-Diphenylpropanoates, 3-Aryl-indole, 4-Aryl-3,4-dihydro-quinolin-2-one and Formal Synthesis of (-)-Indatraline
Taylor, J. G.; Correia, C. R. D.
J. Org. Chem. 2011, 76, 857-869.
Substrate-Directable Heck Reactions with Arenediazonium Salts. The Regio- and Stereoselective Arylation of Allylamine Derivatives and Applications in the Synthesis of Naftifine and Abamines
Prediger, P.; Barbosa, L. F. ; Génisson, Y.; Correia, C. R. D.
J. Org. Chem. 2011, 76, 7737-7749.
Expeditious synthesis of 3,4-dihydroisocoumarins and phthalides using the Heck Matsuda reaction
da Penha, E. T. ; Forni, J. A. ; Biajoli, A. F. P.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 3861-3864.
Heck-Matsuda arylation of 2-hetero-substituted acrylates
de Azambuja, F.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 42-45.
Stereoselective Synthesis of Aza Analogues of Isoaltholactone and Goniothalesdiol - New Applications of the Heck-Matsuda Reaction
Moro, A. V.; dos Santos, M. R.; Correia, C. R. D.Eur.
J. Org. Chem. 2011, 7259-7270.
The first intramolecular Heck Matsuda reaction and its application in the syntheses of benzofurans and indoles
Siqueira, F. A.; Taylor, J. G.; Correia, C. R. D.
Tetrahedron Lett. 2010, 51, 2102-2105.
Synthesis of Novel Room Temperature Chiral Ionic Liquids. Application as Reaction Media for the Heck Arylation of Aza-endocyclic Acrylates
Pastre, J. C.; Genisson, Y.; Saffon, N.; Dandurand, J.; Correia, C. R. D.
J. Braz. Chem. Soc. 2010, 21, 821-836.
Arylation of β,γ-unsaturated lactones by a Heck-Matsuda reaction: an unexpected route to aryldiazene butenolides and pyridazinones
Taylor, J. G.; Correia, C. R. D.
Química Nova 2010, 33, 2070-2074.
The scope of the Heck arylation of enol ethers with arenediazonium salts: a new approach to the synthesis of flavonoids
Machado, A. H. L.; De Sousa, M.; Patto, D.; Azevedo, L.; Bombonato, F. I.; Correia, C. R. D.
Tetrahedron Lett. 2009, 50, 1222-1225.
Remarkable Electronic Effect on the Diastereoselectivity of the Heck Reaction of Methyl Cinnamate with Arenediazonium Salts: Formal Total Synthesis of (±)-Indatraline and (±)-Sertraline
Pastre, J. C.; Correia, C. R. D.
Adv. Synth. Cat. 2009, 351,1217-1223.
Highly Regio- and Stereoselective Heck Reaction of Allylic Esters with Arenediazonium Salts: Application to the Synthesis of Kavalactones
Moro, A. V.; Cardoso, F. S. P.; Correia, C. R. D.
Org. Lett. 2009, 11, 3642-3645.
Heck arylation of styrenes with arenediazonium salts: short, efficient, and stereoselective synthesis of resveratrol, DMU-212, and analogues
Moro, A. V.; Cardoso, Pereira, F. S.; Correia, C. R. D.
Tetrahedron Lett. 2008, 49, 5668-5671
Synthesis of conformationally restricted acetylcholine analogues. Comparing lipase-mediated resolution with simulated moving bed chromatography of arylated β-hydroxy-pyrrolidines
Barreto, R. L.; Carpes, M. J. S.; Santana, C. C.; Correia, C. R. D.
Tetrahedron: Asymmetry 2007, 18, 435-442.
Regio- and Stereoselective Heck Arylations of N -Carbomethoxy- L -3-Dehydroproline Methyl Ester with Arenediazonium Salts. Total Synthesis of Neuroexcitatory Aryl Kainoids
da Silva, K. P.; Godoi, M. N.; Correia, C. R. D.
Org. Lett. 2007, 9, 2815-2818.
Stereoselective Heck-Matsuda Arylations of Chiral Dihydrofurans with Arenediazonium Tetrafluoroborates: An Efficient Enantioselective Total Synthesis of (-)-Isoaltholactone
Meira, P.; Moro, A. V.; Correia, C. R. D.
Synthesis 2007, 2279-2286.
Heck Arylation of Maleic Anhydrides Using Arenediazonium Tetrafluoroborates. Synthesis of Mono and Diarylated Maleic Anhydrides and of the Marine Alkaloids Prepolycitrin A and Polycitrin A
Burtoloso, A. C. B.; Garcia, A. L. L.; Miranda, K. C.; Correia, C. R. D.
Synlett 2006, 18, 3145-3149.
Efficient Heck Arylations of Cyclic and Acyclic Acrylate Derivatives Using Arenediazonium Tetrafluoroborates. A New Synthesis of the Antidepressant Drug (±)-Paroxetine
Pastre, J. C.; Correia, C. R. D.
Org. Lett. 2006, 8, 1657-1660.
Synthesis of 4-Aryl-2-pyrrolidones and β-Aryl-γ-amino-butyric Acid (GABA) Analogues by Heck Arylation of 3-Pyrrolines with Arenediazonium Tetrafluoroborates. Synthesis of (±)-Rolipram on a Multigram Scale and Chromatographic Resolution by Semipreparative Chiral Simulated Moving Bed Chromatography
Garcia, A. L. L.; Carpes, M. J. S.; de Oca, A. C. B. M.; dos Santos, M. A. G.; Santana, C. C.; Correia, C. R. D.
J. Org. Chem. 2005, 70, 1050-1053
Probing the Mechanism of the Heck Reaction with Arene Diazonium Salts by Electrospray Mass and Tandem Mass Spectrometry
Sabino, A. A.; Machado, A. H. L.; Eberlin, M. N.; Correia, C. R. D.
Angew. Chem. Int. Ed. 2004, 43, 2514-2518
Probing the Stereoselectivity of the Heck Arylation of Endocyclic Enecarbamates with Diazonium Salts. Concise Syntheses of (2S, 5R)-Phenylproline Methyl Ester and Schramm’s C-Azanucleoside
Severino, E. A.; Costenaro, E. R.; Garcia, A. L. L.; Correia, C. R. D.
Org. Lett. 2003, 5, 305-308
Synthesis of Aryl Pyrrolizidines from Endocyclic Enecarbamates. Novel Applications of the Heck Arylation of 3-Pyrrolines Using Diazonium Salts
Montes de Oca, A. C. B.; Correia, C. R. D.
ARKIVOC 2003, 10, 390-403
Heck arylations of N-acyl-3-pyrroline an N-acyl-1,2,5,6-tetrahydropyridine with aryldiazonium salts. Short syntheses of aryl y-and -lactams, baclofen, homobaclofen and analogues
Carpes, M. J. S.; Correia, C. R. D.
Tetrahedron Lett. 2002, 43, 741-744
Heck Arylation of N-Boc-3-Pyrrolines and N-Boc-2-Pyrrolines with Diazonium Salts. Efficient Syntheses of Five-Membered 4-Aryl Endocyclic Enecarbamates and N-Boc-2,4-Diaryl 3-Pyrrolines
Carpes, M. J. S.; Correia, C. R. D.
Synlett 2000, 1037-1039.
Heck Arylation of Endocyclic Enecarbamates with Diazonium Salts. Improvements and a Concise Enantioselective Synthesis of (-)-Codonopsinine
Severino, E. A.; Correia, C. R. D.
Org. Lett. 2000, 2, 3039-3042
Heck Reaction of Endocyclic Enecarbamates with Diazonium Salts. Formal Enantioselective Synthesis of Alkaloids (-)-Codonopsine and (-)-Codonopsinine, and the Synthesis of a New C-aryl Aza-Sugar
Oliveira, D. F.; Severino, E. A.; Correia, C. R. D.
Tetrahedron Lett. 1999, 40, 2083-2086
Enantioselective Synthesis of Phthalides and Isochromanones via Heck–Matsuda Arylation of Dihydrofurans
Kattela, S.; de Lucca, E. C., Jr. ; Correia, C. R. D
Chem. Eur. J., 2018, 24, 11691-11696.
N,N‐Chiral Ligands Enabling Palladium‐catalyzed Enantioselective Intramolecular Heck‐Matsuda Carbonylation Reactions by Sequential Migratory and CO Insertions
Carmona, R. C.; Köster, O. D.; Correia, C. R. D.
Angew. Chem. Int. Ed., 2018, 130, 12243-12246.
Non-Covalent Substrate Directed Enantioselective Heck Desymmetrization of cis-Cyclohex-4-ene-1,2-diol: Synthesis of All cis Chiral 5-Aryl-cyclohex-3-ene-1,2-diols and Mechanistic Investigation
Angnes, R. A.; Thompson, L. M.; Mashuta, M. S.; Correia, C. R. D.; Hammond, G. B.,
Adv. Synth. Cat., 2018, 360, 3760-3767.
Enantioselective, Noncovalent, Substrate-Directable Heck–Matsuda and Oxidative Heck Arylations of Unactivated Five-Membered Carbocyclic Olefins
Oliveira, J. M.; Angnes, R. A.; Khan, I. K.; Polo, E. C.; Heerdt, G.; Servilha, B. M.; Silva, V. H. M.; Braga, A. A. C.; Correia, C. R. D
Chem. Eur. J., 2018, 24, 11738-11747.
Regioselective and Stereoselective Heck–Matsuda Arylations of Trisubstituted Allylic Alkenols and Their Silyl and Methyl Ether Derivatives To Access Two Contiguous Stereogenic Centers: Expanding the Redox-Relay Process and Application in the Total Synthesis of meso-Hexestrol
Frota, C.; Polo, E. C.; Esteves, H.; Correia, C. R. D.
J. Org. Chem. 2018, 83, 2198–2209.
EnantioselectiveOxy-Heck–Matsuda Arylations:ExpeditiousSynthesis of Dihydrobenzofuran Systems and Total Synthesis ofthe Neolignan (-)-Conocarpan
Silva, A. R.; Polo, E. C.; Martins, N. M.; Correia, C. R. D.
Adv. Synth. Cat., 2018, 360, 346-365.
Non-Covalent Carbonyl-Directed Heck–Matsuda Desymmetrizations: Synthesis of Cyclopentene-Fused Spirooxindoles, Spirolactones, and Spirolactams
Kattela, S.; Heerdt, G.; Correia, C. R. D.
Adv. Synth. Cat., 2017, 260-267.
Enantioselective total synthesis of the highly selective sphingosine-1-receptor VPC01091 by the Heck desymmetrization of a non-activated cyclopentene-fused spiro-pyrrolidinone
Khan, I. U.; Kattela, S.; Hassan, A.; Correia, C. R. D.
Org. Biomol. Chem., 2016,14, 9476-9480.
Non-Covalent Substrate-Directed Enantioselective heck Reactions. Synthesis of S- and P-Stereogenic Heterocycles
de Azambuja, F.; Carmona, R. C.; Chorro, T.; Heerdt, G.; Correia, C. R. D.
Chem. Eur. J. 2016, 22, 11205-11209.
Intermolecular Noncovalent Hydroxy-Directed Enantioselective Heck Desymmetrization of Cyclopentenol: Computationally Driven Synthesis of Highly Functionalized cis-4-Arylcyclopentenol Scaffolds
Oliveira, J. S.; Angnes, R. A.; Silva, V. H. M.; Servilha, B. M.; Adeel, M.; Braga, A. A. C.; Aponick, A.; Correia, C. R. D.
J. Org. Chem. 2016, 81, 2010–2018.
Quaternary Stereogenic Centers through Enantioselective Heck Arylation of Acyclic Olefins with Aryldiazonium Salts: Application in a Concise Synthesis of (R)-Verapamil
Oliveira, C. C.; Pfaltz, A.; Correia, C. R. D.
Angew. Chem. Int. Ed. 2015, 54, 14036-14039.
Stereoselective Synthesis of 3-Hydroxy-4-arylcyclopentanones and 4-Arylcyclopentenones through a Heck–Matsuda Desymmetrization of meso cis-4-Cyclopentene-1,3-diol
Carmona, R. C. ; Correia, C. R. D.
Adv. Synth. Cat. 2015, 357, 2639-2643.
Finelli, F. G. ; Godoi, M. N. ; Correia, C. R. D.
J. Braz. Chem. Soc. 2015, 26, 910-915.
Chemo-, Regio- and Stereoselective Heck Arylation of Allylated Malonates: Mechanistic Insights by ESI-MS and Synthetic Application toward 5-Arylmethyl-γ-lactones
Oliveira, C. C. ; Marques, M. V. ; Godoi, M. N. ; Regiani, T. ; Santos, V. G. ; dos Santos E. A. F. ; Eberlin, M. N. ; Sá, M. M. ; Correia, C. R. D.
Org. Lett. 2014, 16, 5180-5183.
Improved synthesis of bioactive stilbene derivatives applying design of experiments to the Heck–Matsuda reaction
Perez, C. C. ; Pena, J. M. ; Correia, C. R. D.
New J. Chem. 2014, 38, 3933-3938.
Stereoselective Synthesis of Aryl Cyclopentene Scaffolds by Heck–Matsuda Desymmetrization of 3-Cyclopentenol
Angnes, R. A. ; Oliveira, J. M. ; O., Caio C. ; Martins, N. C. ; Correia, C. R. D.
Chem. Eur. J. 2014, 20, 13117-13121.
Fluorescence from bisaryl-substituted maleimide derivatives
Lauer, M. H.; Drekener, R. L.; Correia, C. R. D.; Gehlen, M. H.
Photochem. Photobiol. Sci. 2014, 13, 859-866.
Construction of 3-arylpropylamines using Heck arylations. The total synthesis of cinacalcet hydrochloride, alverine, and tolpropamine
Prediger, P.; Silva, A. R.; Correia, C. R. D.
Tetrahedron. 2014, 70, 3333-3341.
Expeditious Synthesis of the Marine Natural Products Prepolycitrin A and Polycitrins A and B through Heck Arylations
Canto, K.; Ribeiro, R. S.; Biajoli, A. F. P.; Correia, C. R. D.
Eur. J. Org Chem. 2013, 35, 8004-8013.
“Dba-free” palladium intermediates of the Heck–Matsuda reaction
Machado, A. H. L.; Milagre, H. M. S.; Eberlin, L. S.; Sabino A. A.; Correia, C. R. D.; Eberlin, M. N.
Org. Biomol. Chem. 2013,11, 3277-3281.
Ionic Liquids and the Heck Coupling Reaction: An Update
Prediger, P.; Genisson, Y.; Correia, C. R. D.
Curr. Org. Chem. 2013, 17, 238-256.
Intermolecular Enantioselective Heck–Matsuda Arylations of Acyclic Olefins: Application to the Synthesis of β-Aryl-γ-lactones and β-Aryl Aldehydes
Oliveira, C. C.; Angnes, R. A. ;Correia, C. R. D.
J. Org. Chem. 2013, 78, 4373−4385.
Synthesis of pentabromopseudilin and other arylpyrrole derivatives via Heck arylations
Schwalm, C. S.; de Castro, I. B. D.; Ferrari, J. ; de Oliveira, F. L.; Aparicio, R. ;Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 1660-1663.
The first examples of the enantioselective Heck Matsuda reaction: arylation of unactivated cyclic olefins using chiral bisoxazolines
Oliveira, C. C.; Salles, A. G.; Santos, E. A. F.; Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 3325-3328.
Heck-Matsuda Arylation as a Strategy to Access Kavalactones Isolated from Polygala sabulosa
Soldi, C.; Moro, A. V.; Pizzolatti, M. G.; Correia, C. R. D.
Eur. J. Org Chem. 2012, 3607-3616.
Divergent total synthesis of the natural antimalarial marinoquinolines A, B, C, E and unnatural analogues
Schwalm, C. S.; Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 4836-4840.
Palladium catalysed regioselective arylation of indoles, benzofuran and benzothiophene with aryldiazonium salts
Baijoli, A. F. P.; da Penha, E. T.; Correia, C. R. D.
RSC Advances 2012, 2, 11930.
Coumarins from Free ortho-Hydroxy Cinnamates by Heck-Matsuda Arylations: A Scalable Total Synthesis of (R)-Tolterodine
Barancelli, D. A.; Salles, A. G. ; Taylor, J. G.; Correia, C. R. D.
Org. Lett. 2012, 14, 6036-6039.
Stereoselective Arylation of Substituted Cyclopentenes by Substrate-Directable Heck Matsuda Reactions: A Concise Total Synthesis of the Sphingosine 1-Phosphate Receptor (S1P) Agonist VPC01091
Oliveira, C. C. ; dos Santos, E. A. F. ; Nunes, J. H. B.; Correia, C. R. D.
J. Org. Chem. 2012, 77, 8182-8190.
Evolution and Synthetic Applications of the Heck-Matsuda Reaction: The Return of Arenediazonium Salts to Prominence
Taylor, J. G.; Moro, A. V.; Correia, C. R. D.
Eur. J. Org Chem. 2011, 1403-1428.This revision article was in the top-25 most accessed in European Journal of Organic Chemistry in 2011-2012
Facile synthesis of symmetrical 3,3-diarylacrylates by a Heck-Matsuda reaction: an expedient route to biologically active indanones
Taylor, J. G.; Ribeiro, R. S.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 3861-3864.
Stereoselective Synthesis of Unsymmetrical β,β-Diarylacrylates by a Heck-Matsuda Reaction: Versatile Building Blocks for Asymmetric Synthesis of β,β-Diphenylpropanoates, 3-Aryl-indole, 4-Aryl-3,4-dihydro-quinolin-2-one and Formal Synthesis of (-)-Indatraline
Taylor, J. G.; Correia, C. R. D.
J. Org. Chem. 2011, 76, 857-869.
Substrate-Directable Heck Reactions with Arenediazonium Salts. The Regio- and Stereoselective Arylation of Allylamine Derivatives and Applications in the Synthesis of Naftifine and Abamines
Prediger, P.; Barbosa, L. F. ; Génisson, Y.; Correia, C. R. D.
J. Org. Chem. 2011, 76, 7737-7749.
Expeditious synthesis of 3,4-dihydroisocoumarins and phthalides using the Heck Matsuda reaction
da Penha, E. T. ; Forni, J. A. ; Biajoli, A. F. P.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 3861-3864.
Heck-Matsuda arylation of 2-hetero-substituted acrylates
de Azambuja, F.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 42-45.
Stereoselective Synthesis of Aza Analogues of Isoaltholactone and Goniothalesdiol - New Applications of the Heck-Matsuda Reaction
Moro, A. V.; dos Santos, M. R.; Correia, C. R. D.Eur.
J. Org. Chem. 2011, 7259-7270.
The first intramolecular Heck Matsuda reaction and its application in the syntheses of benzofurans and indoles
Siqueira, F. A.; Taylor, J. G.; Correia, C. R. D.
Tetrahedron Lett. 2010, 51, 2102-2105.
Synthesis of Novel Room Temperature Chiral Ionic Liquids. Application as Reaction Media for the Heck Arylation of Aza-endocyclic Acrylates
Pastre, J. C.; Genisson, Y.; Saffon, N.; Dandurand, J.; Correia, C. R. D.
J. Braz. Chem. Soc. 2010, 21, 821-836.
Arylation of β,γ-unsaturated lactones by a Heck-Matsuda reaction: an unexpected route to aryldiazene butenolides and pyridazinones
Taylor, J. G.; Correia, C. R. D.
Química Nova 2010, 33, 2070-2074.
The scope of the Heck arylation of enol ethers with arenediazonium salts: a new approach to the synthesis of flavonoids
Machado, A. H. L.; De Sousa, M.; Patto, D.; Azevedo, L.; Bombonato, F. I.; Correia, C. R. D.
Tetrahedron Lett. 2009, 50, 1222-1225.
Remarkable Electronic Effect on the Diastereoselectivity of the Heck Reaction of Methyl Cinnamate with Arenediazonium Salts: Formal Total Synthesis of (±)-Indatraline and (±)-Sertraline
Pastre, J. C.; Correia, C. R. D.
Adv. Synth. Cat. 2009, 351,1217-1223.
Highly Regio- and Stereoselective Heck Reaction of Allylic Esters with Arenediazonium Salts: Application to the Synthesis of Kavalactones
Moro, A. V.; Cardoso, F. S. P.; Correia, C. R. D.
Org. Lett. 2009, 11, 3642-3645.
Heck arylation of styrenes with arenediazonium salts: short, efficient, and stereoselective synthesis of resveratrol, DMU-212, and analogues
Moro, A. V.; Cardoso, Pereira, F. S.; Correia, C. R. D.
Tetrahedron Lett. 2008, 49, 5668-5671
Synthesis of conformationally restricted acetylcholine analogues. Comparing lipase-mediated resolution with simulated moving bed chromatography of arylated β-hydroxy-pyrrolidines
Barreto, R. L.; Carpes, M. J. S.; Santana, C. C.; Correia, C. R. D.
Tetrahedron: Asymmetry 2007, 18, 435-442.
Regio- and Stereoselective Heck Arylations of N -Carbomethoxy- L -3-Dehydroproline Methyl Ester with Arenediazonium Salts. Total Synthesis of Neuroexcitatory Aryl Kainoids
da Silva, K. P.; Godoi, M. N.; Correia, C. R. D.
Org. Lett. 2007, 9, 2815-2818.
Stereoselective Heck-Matsuda Arylations of Chiral Dihydrofurans with Arenediazonium Tetrafluoroborates: An Efficient Enantioselective Total Synthesis of (-)-Isoaltholactone
Meira, P.; Moro, A. V.; Correia, C. R. D.
Synthesis 2007, 2279-2286.
Heck Arylation of Maleic Anhydrides Using Arenediazonium Tetrafluoroborates. Synthesis of Mono and Diarylated Maleic Anhydrides and of the Marine Alkaloids Prepolycitrin A and Polycitrin A
Burtoloso, A. C. B.; Garcia, A. L. L.; Miranda, K. C.; Correia, C. R. D.
Synlett 2006, 18, 3145-3149.
Efficient Heck Arylations of Cyclic and Acyclic Acrylate Derivatives Using Arenediazonium Tetrafluoroborates. A New Synthesis of the Antidepressant Drug (±)-Paroxetine
Pastre, J. C.; Correia, C. R. D.
Org. Lett. 2006, 8, 1657-1660.
Synthesis of 4-Aryl-2-pyrrolidones and β-Aryl-γ-amino-butyric Acid (GABA) Analogues by Heck Arylation of 3-Pyrrolines with Arenediazonium Tetrafluoroborates. Synthesis of (±)-Rolipram on a Multigram Scale and Chromatographic Resolution by Semipreparative Chiral Simulated Moving Bed Chromatography
Garcia, A. L. L.; Carpes, M. J. S.; de Oca, A. C. B. M.; dos Santos, M. A. G.; Santana, C. C.; Correia, C. R. D.
J. Org. Chem. 2005, 70, 1050-1053
Probing the Mechanism of the Heck Reaction with Arene Diazonium Salts by Electrospray Mass and Tandem Mass Spectrometry
Sabino, A. A.; Machado, A. H. L.; Eberlin, M. N.; Correia, C. R. D.
Angew. Chem. Int. Ed. 2004, 43, 2514-2518
Probing the Stereoselectivity of the Heck Arylation of Endocyclic Enecarbamates with Diazonium Salts. Concise Syntheses of (2S, 5R)-Phenylproline Methyl Ester and Schramm’s C-Azanucleoside
Severino, E. A.; Costenaro, E. R.; Garcia, A. L. L.; Correia, C. R. D.
Org. Lett. 2003, 5, 305-308
Synthesis of Aryl Pyrrolizidines from Endocyclic Enecarbamates. Novel Applications of the Heck Arylation of 3-Pyrrolines Using Diazonium Salts
Montes de Oca, A. C. B.; Correia, C. R. D.
ARKIVOC 2003, 10, 390-403
Heck arylations of N-acyl-3-pyrroline an N-acyl-1,2,5,6-tetrahydropyridine with aryldiazonium salts. Short syntheses of aryl y-and -lactams, baclofen, homobaclofen and analogues
Carpes, M. J. S.; Correia, C. R. D.
Tetrahedron Lett. 2002, 43, 741-744
Heck Arylation of N-Boc-3-Pyrrolines and N-Boc-2-Pyrrolines with Diazonium Salts. Efficient Syntheses of Five-Membered 4-Aryl Endocyclic Enecarbamates and N-Boc-2,4-Diaryl 3-Pyrrolines
Carpes, M. J. S.; Correia, C. R. D.
Synlett 2000, 1037-1039.
Heck Arylation of Endocyclic Enecarbamates with Diazonium Salts. Improvements and a Concise Enantioselective Synthesis of (-)-Codonopsinine
Severino, E. A.; Correia, C. R. D.
Org. Lett. 2000, 2, 3039-3042
Heck Reaction of Endocyclic Enecarbamates with Diazonium Salts. Formal Enantioselective Synthesis of Alkaloids (-)-Codonopsine and (-)-Codonopsinine, and the Synthesis of a New C-aryl Aza-Sugar
Oliveira, D. F.; Severino, E. A.; Correia, C. R. D.
Tetrahedron Lett. 1999, 40, 2083-2086
Enantioselective Synthesis of Phthalides and Isochromanones via Heck–Matsuda Arylation of Dihydrofurans
Kattela, S.; de Lucca, E. C., Jr. ; Correia, C. R. D
Chem. Eur. J., 2018, 24, 11691-11696.
N,N‐Chiral Ligands Enabling Palladium‐catalyzed Enantioselective Intramolecular Heck‐Matsuda Carbonylation Reactions by Sequential Migratory and CO Insertions
Carmona, R. C.; Köster, O. D.; Correia, C. R. D.
Angew. Chem. Int. Ed., 2018, 130, 12243-12246.
Non-Covalent Substrate Directed Enantioselective Heck Desymmetrization of cis-Cyclohex-4-ene-1,2-diol: Synthesis of All cis Chiral 5-Aryl-cyclohex-3-ene-1,2-diols and Mechanistic Investigation
Angnes, R. A.; Thompson, L. M.; Mashuta, M. S.; Correia, C. R. D.; Hammond, G. B.,
Adv. Synth. Cat., 2018, 360, 3760-3767.
Enantioselective, Noncovalent, Substrate-Directable Heck–Matsuda and Oxidative Heck Arylations of Unactivated Five-Membered Carbocyclic Olefins
Oliveira, J. M.; Angnes, R. A.; Khan, I. K.; Polo, E. C.; Heerdt, G.; Servilha, B. M.; Silva, V. H. M.; Braga, A. A. C.; Correia, C. R. D
Chem. Eur. J., 2018, 24, 11738-11747.
Regioselective and Stereoselective Heck–Matsuda Arylations of Trisubstituted Allylic Alkenols and Their Silyl and Methyl Ether Derivatives To Access Two Contiguous Stereogenic Centers: Expanding the Redox-Relay Process and Application in the Total Synthesis of meso-Hexestrol
Frota, C.; Polo, E. C.; Esteves, H.; Correia, C. R. D.
J. Org. Chem. 2018, 83, 2198–2209.
EnantioselectiveOxy-Heck–Matsuda Arylations:ExpeditiousSynthesis of Dihydrobenzofuran Systems and Total Synthesis ofthe Neolignan (-)-Conocarpan
Silva, A. R.; Polo, E. C.; Martins, N. M.; Correia, C. R. D.
Adv. Synth. Cat., 2018, 360, 346-365.
Non-Covalent Carbonyl-Directed Heck–Matsuda Desymmetrizations: Synthesis of Cyclopentene-Fused Spirooxindoles, Spirolactones, and Spirolactams
Kattela, S.; Heerdt, G.; Correia, C. R. D.
Adv. Synth. Cat., 2017, 260-267.
Enantioselective total synthesis of the highly selective sphingosine-1-receptor VPC01091 by the Heck desymmetrization of a non-activated cyclopentene-fused spiro-pyrrolidinone
Khan, I. U.; Kattela, S.; Hassan, A.; Correia, C. R. D.
Org. Biomol. Chem., 2016,14, 9476-9480.
Non-Covalent Substrate-Directed Enantioselective heck Reactions. Synthesis of S- and P-Stereogenic Heterocycles
de Azambuja, F.; Carmona, R. C.; Chorro, T.; Heerdt, G.; Correia, C. R. D.
Chem. Eur. J. 2016, 22, 11205-11209.
Intermolecular Noncovalent Hydroxy-Directed Enantioselective Heck Desymmetrization of Cyclopentenol: Computationally Driven Synthesis of Highly Functionalized cis-4-Arylcyclopentenol Scaffolds
Oliveira, J. S.; Angnes, R. A.; Silva, V. H. M.; Servilha, B. M.; Adeel, M.; Braga, A. A. C.; Aponick, A.; Correia, C. R. D.
J. Org. Chem. 2016, 81, 2010–2018.
Quaternary Stereogenic Centers through Enantioselective Heck Arylation of Acyclic Olefins with Aryldiazonium Salts: Application in a Concise Synthesis of (R)-Verapamil
Oliveira, C. C.; Pfaltz, A.; Correia, C. R. D.
Angew. Chem. Int. Ed. 2015, 54, 14036-14039.
Stereoselective Synthesis of 3-Hydroxy-4-arylcyclopentanones and 4-Arylcyclopentenones through a Heck–Matsuda Desymmetrization of meso cis-4-Cyclopentene-1,3-diol
Carmona, R. C. ; Correia, C. R. D.
Adv. Synth. Cat. 2015, 357, 2639-2643.
Finelli, F. G. ; Godoi, M. N. ; Correia, C. R. D.
J. Braz. Chem. Soc. 2015, 26, 910-915.
Chemo-, Regio- and Stereoselective Heck Arylation of Allylated Malonates: Mechanistic Insights by ESI-MS and Synthetic Application toward 5-Arylmethyl-γ-lactones
Oliveira, C. C. ; Marques, M. V. ; Godoi, M. N. ; Regiani, T. ; Santos, V. G. ; dos Santos E. A. F. ; Eberlin, M. N. ; Sá, M. M. ; Correia, C. R. D.
Org. Lett. 2014, 16, 5180-5183.
Improved synthesis of bioactive stilbene derivatives applying design of experiments to the Heck–Matsuda reaction
Perez, C. C. ; Pena, J. M. ; Correia, C. R. D.
New J. Chem. 2014, 38, 3933-3938.
Stereoselective Synthesis of Aryl Cyclopentene Scaffolds by Heck–Matsuda Desymmetrization of 3-Cyclopentenol
Angnes, R. A. ; Oliveira, J. M. ; O., Caio C. ; Martins, N. C. ; Correia, C. R. D.
Chem. Eur. J. 2014, 20, 13117-13121.
Fluorescence from bisaryl-substituted maleimide derivatives
Lauer, M. H.; Drekener, R. L.; Correia, C. R. D.; Gehlen, M. H.
Photochem. Photobiol. Sci. 2014, 13, 859-866.
Construction of 3-arylpropylamines using Heck arylations. The total synthesis of cinacalcet hydrochloride, alverine, and tolpropamine
Prediger, P.; Silva, A. R.; Correia, C. R. D.
Tetrahedron. 2014, 70, 3333-3341.
Expeditious Synthesis of the Marine Natural Products Prepolycitrin A and Polycitrins A and B through Heck Arylations
Canto, K.; Ribeiro, R. S.; Biajoli, A. F. P.; Correia, C. R. D.
Eur. J. Org Chem. 2013, 35, 8004-8013.
“Dba-free” palladium intermediates of the Heck–Matsuda reaction
Machado, A. H. L.; Milagre, H. M. S.; Eberlin, L. S.; Sabino A. A.; Correia, C. R. D.; Eberlin, M. N.
Org. Biomol. Chem. 2013,11, 3277-3281.
Ionic Liquids and the Heck Coupling Reaction: An Update
Prediger, P.; Genisson, Y.; Correia, C. R. D.
Curr. Org. Chem. 2013, 17, 238-256.
Intermolecular Enantioselective Heck–Matsuda Arylations of Acyclic Olefins: Application to the Synthesis of β-Aryl-γ-lactones and β-Aryl Aldehydes
Oliveira, C. C.; Angnes, R. A. ;Correia, C. R. D.
J. Org. Chem. 2013, 78, 4373−4385.
Synthesis of pentabromopseudilin and other arylpyrrole derivatives via Heck arylations
Schwalm, C. S.; de Castro, I. B. D.; Ferrari, J. ; de Oliveira, F. L.; Aparicio, R. ;Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 1660-1663.
The first examples of the enantioselective Heck Matsuda reaction: arylation of unactivated cyclic olefins using chiral bisoxazolines
Oliveira, C. C.; Salles, A. G.; Santos, E. A. F.; Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 3325-3328.
Heck-Matsuda Arylation as a Strategy to Access Kavalactones Isolated from Polygala sabulosa
Soldi, C.; Moro, A. V.; Pizzolatti, M. G.; Correia, C. R. D.
Eur. J. Org Chem. 2012, 3607-3616.
Divergent total synthesis of the natural antimalarial marinoquinolines A, B, C, E and unnatural analogues
Schwalm, C. S.; Correia, C. R. D.
Tetrahedron Lett. 2012, 53, 4836-4840.
Palladium catalysed regioselective arylation of indoles, benzofuran and benzothiophene with aryldiazonium salts
Baijoli, A. F. P.; da Penha, E. T.; Correia, C. R. D.
RSC Advances 2012, 2, 11930.
Coumarins from Free ortho-Hydroxy Cinnamates by Heck-Matsuda Arylations: A Scalable Total Synthesis of (R)-Tolterodine
Barancelli, D. A.; Salles, A. G. ; Taylor, J. G.; Correia, C. R. D.
Org. Lett. 2012, 14, 6036-6039.
Stereoselective Arylation of Substituted Cyclopentenes by Substrate-Directable Heck Matsuda Reactions: A Concise Total Synthesis of the Sphingosine 1-Phosphate Receptor (S1P) Agonist VPC01091
Oliveira, C. C. ; dos Santos, E. A. F. ; Nunes, J. H. B.; Correia, C. R. D.
J. Org. Chem. 2012, 77, 8182-8190.
Evolution and Synthetic Applications of the Heck-Matsuda Reaction: The Return of Arenediazonium Salts to Prominence
Taylor, J. G.; Moro, A. V.; Correia, C. R. D.
Eur. J. Org Chem. 2011, 1403-1428.This revision article was in the top-25 most accessed in European Journal of Organic Chemistry in 2011-2012
Facile synthesis of symmetrical 3,3-diarylacrylates by a Heck-Matsuda reaction: an expedient route to biologically active indanones
Taylor, J. G.; Ribeiro, R. S.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 3861-3864.
Stereoselective Synthesis of Unsymmetrical β,β-Diarylacrylates by a Heck-Matsuda Reaction: Versatile Building Blocks for Asymmetric Synthesis of β,β-Diphenylpropanoates, 3-Aryl-indole, 4-Aryl-3,4-dihydro-quinolin-2-one and Formal Synthesis of (-)-Indatraline
Taylor, J. G.; Correia, C. R. D.
J. Org. Chem. 2011, 76, 857-869.
Substrate-Directable Heck Reactions with Arenediazonium Salts. The Regio- and Stereoselective Arylation of Allylamine Derivatives and Applications in the Synthesis of Naftifine and Abamines
Prediger, P.; Barbosa, L. F. ; Génisson, Y.; Correia, C. R. D.
J. Org. Chem. 2011, 76, 7737-7749.
Expeditious synthesis of 3,4-dihydroisocoumarins and phthalides using the Heck Matsuda reaction
da Penha, E. T. ; Forni, J. A. ; Biajoli, A. F. P.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 3861-3864.
Heck-Matsuda arylation of 2-hetero-substituted acrylates
de Azambuja, F.; Correia, C. R. D.
Tetrahedron Lett. 2011, 52, 42-45.
Stereoselective Synthesis of Aza Analogues of Isoaltholactone and Goniothalesdiol - New Applications of the Heck-Matsuda Reaction
Moro, A. V.; dos Santos, M. R.; Correia, C. R. D.Eur.
J. Org. Chem. 2011, 7259-7270.
The first intramolecular Heck Matsuda reaction and its application in the syntheses of benzofurans and indoles
Siqueira, F. A.; Taylor, J. G.; Correia, C. R. D.
Tetrahedron Lett. 2010, 51, 2102-2105.
Synthesis of Novel Room Temperature Chiral Ionic Liquids. Application as Reaction Media for the Heck Arylation of Aza-endocyclic Acrylates
Pastre, J. C.; Genisson, Y.; Saffon, N.; Dandurand, J.; Correia, C. R. D.
J. Braz. Chem. Soc. 2010, 21, 821-836.
Arylation of β,γ-unsaturated lactones by a Heck-Matsuda reaction: an unexpected route to aryldiazene butenolides and pyridazinones
Taylor, J. G.; Correia, C. R. D.
Química Nova 2010, 33, 2070-2074.
The scope of the Heck arylation of enol ethers with arenediazonium salts: a new approach to the synthesis of flavonoids
Machado, A. H. L.; De Sousa, M.; Patto, D.; Azevedo, L.; Bombonato, F. I.; Correia, C. R. D.
Tetrahedron Lett. 2009, 50, 1222-1225.
Remarkable Electronic Effect on the Diastereoselectivity of the Heck Reaction of Methyl Cinnamate with Arenediazonium Salts: Formal Total Synthesis of (±)-Indatraline and (±)-Sertraline
Pastre, J. C.; Correia, C. R. D.
Adv. Synth. Cat. 2009, 351,1217-1223.
Highly Regio- and Stereoselective Heck Reaction of Allylic Esters with Arenediazonium Salts: Application to the Synthesis of Kavalactones
Moro, A. V.; Cardoso, F. S. P.; Correia, C. R. D.
Org. Lett. 2009, 11, 3642-3645.
Heck arylation of styrenes with arenediazonium salts: short, efficient, and stereoselective synthesis of resveratrol, DMU-212, and analogues
Moro, A. V.; Cardoso, Pereira, F. S.; Correia, C. R. D.
Tetrahedron Lett. 2008, 49, 5668-5671
Synthesis of conformationally restricted acetylcholine analogues. Comparing lipase-mediated resolution with simulated moving bed chromatography of arylated β-hydroxy-pyrrolidines
Barreto, R. L.; Carpes, M. J. S.; Santana, C. C.; Correia, C. R. D.
Tetrahedron: Asymmetry 2007, 18, 435-442.
Regio- and Stereoselective Heck Arylations of N -Carbomethoxy- L -3-Dehydroproline Methyl Ester with Arenediazonium Salts. Total Synthesis of Neuroexcitatory Aryl Kainoids
da Silva, K. P.; Godoi, M. N.; Correia, C. R. D.
Org. Lett. 2007, 9, 2815-2818.
Stereoselective Heck-Matsuda Arylations of Chiral Dihydrofurans with Arenediazonium Tetrafluoroborates: An Efficient Enantioselective Total Synthesis of (-)-Isoaltholactone
Meira, P.; Moro, A. V.; Correia, C. R. D.
Synthesis 2007, 2279-2286.
Heck Arylation of Maleic Anhydrides Using Arenediazonium Tetrafluoroborates. Synthesis of Mono and Diarylated Maleic Anhydrides and of the Marine Alkaloids Prepolycitrin A and Polycitrin A
Burtoloso, A. C. B.; Garcia, A. L. L.; Miranda, K. C.; Correia, C. R. D.
Synlett 2006, 18, 3145-3149.
Efficient Heck Arylations of Cyclic and Acyclic Acrylate Derivatives Using Arenediazonium Tetrafluoroborates. A New Synthesis of the Antidepressant Drug (±)-Paroxetine
Pastre, J. C.; Correia, C. R. D.
Org. Lett. 2006, 8, 1657-1660.
Synthesis of 4-Aryl-2-pyrrolidones and β-Aryl-γ-amino-butyric Acid (GABA) Analogues by Heck Arylation of 3-Pyrrolines with Arenediazonium Tetrafluoroborates. Synthesis of (±)-Rolipram on a Multigram Scale and Chromatographic Resolution by Semipreparative Chiral Simulated Moving Bed Chromatography
Garcia, A. L. L.; Carpes, M. J. S.; de Oca, A. C. B. M.; dos Santos, M. A. G.; Santana, C. C.; Correia, C. R. D.
J. Org. Chem. 2005, 70, 1050-1053
Probing the Mechanism of the Heck Reaction with Arene Diazonium Salts by Electrospray Mass and Tandem Mass Spectrometry
Sabino, A. A.; Machado, A. H. L.; Eberlin, M. N.; Correia, C. R. D.
Angew. Chem. Int. Ed. 2004, 43, 2514-2518
Probing the Stereoselectivity of the Heck Arylation of Endocyclic Enecarbamates with Diazonium Salts. Concise Syntheses of (2S, 5R)-Phenylproline Methyl Ester and Schramm’s C-Azanucleoside
Severino, E. A.; Costenaro, E. R.; Garcia, A. L. L.; Correia, C. R. D.
Org. Lett. 2003, 5, 305-308
Synthesis of Aryl Pyrrolizidines from Endocyclic Enecarbamates. Novel Applications of the Heck Arylation of 3-Pyrrolines Using Diazonium Salts
Montes de Oca, A. C. B.; Correia, C. R. D.
ARKIVOC 2003, 10, 390-403
Heck arylations of N-acyl-3-pyrroline an N-acyl-1,2,5,6-tetrahydropyridine with aryldiazonium salts. Short syntheses of aryl y-and -lactams, baclofen, homobaclofen and analogues
Carpes, M. J. S.; Correia, C. R. D.
Tetrahedron Lett. 2002, 43, 741-744
Heck Arylation of N-Boc-3-Pyrrolines and N-Boc-2-Pyrrolines with Diazonium Salts. Efficient Syntheses of Five-Membered 4-Aryl Endocyclic Enecarbamates and N-Boc-2,4-Diaryl 3-Pyrrolines
Carpes, M. J. S.; Correia, C. R. D.
Synlett 2000, 1037-1039.
Heck Arylation of Endocyclic Enecarbamates with Diazonium Salts. Improvements and a Concise Enantioselective Synthesis of (-)-Codonopsinine
Severino, E. A.; Correia, C. R. D.
Org. Lett. 2000, 2, 3039-3042
Heck Reaction of Endocyclic Enecarbamates with Diazonium Salts. Formal Enantioselective Synthesis of Alkaloids (-)-Codonopsine and (-)-Codonopsinine, and the Synthesis of a New C-aryl Aza-Sugar
Oliveira, D. F.; Severino, E. A.; Correia, C. R. D.
Tetrahedron Lett. 1999, 40, 2083-2086